- Research
- Open access
- Published:
The core root microbiome of Spartina alterniflora is predominated by sulfur-oxidizing and sulfate-reducing bacteria in Georgia salt marshes, USA
Microbiome volume 10, Article number: 37 (2022)
Abstract
Background
Salt marshes are dominated by the smooth cordgrass Spartina alterniflora on the US Atlantic and Gulf of Mexico coastlines. Although soil microorganisms are well known to mediate important biogeochemical cycles in salt marshes, little is known about the role of root microbiomes in supporting the health and productivity of marsh plant hosts. Leveraging in situ gradients in aboveground plant biomass as a natural laboratory, we investigated the relationships between S. alterniflora primary productivity, sediment redox potential, and the physiological ecology of bulk sediment, rhizosphere, and root microbial communities at two Georgia barrier islands over two growing seasons.
Results
A marked decrease in prokaryotic alpha diversity with high abundance and increased phylogenetic dispersion was found in the S. alterniflora root microbiome. Significantly higher rates of enzymatic organic matter decomposition, as well as the relative abundances of putative sulfur (S)-oxidizing, sulfate-reducing, and nitrifying prokaryotes correlated with plant productivity. Moreover, these functional guilds were overrepresented in the S. alterniflora rhizosphere and root core microbiomes. Core microbiome bacteria from the Candidatus Thiodiazotropha genus, with the metabolic potential to couple S oxidation with C and N fixation, were shown to be highly abundant in the root and rhizosphere of S. alterniflora.
Conclusions
The S. alterniflora root microbiome is dominated by highly active and competitive species taking advantage of available carbon substrates in the oxidized root zone. Two microbially mediated mechanisms are proposed to stimulate S. alterniflora primary productivity: (i) enhanced microbial activity replenishes nutrients and terminal electron acceptors in higher biomass stands, and (ii) coupling of chemolithotrophic S oxidation with carbon (C) and nitrogen (N) fixation by root- and rhizosphere-associated prokaryotes detoxifies sulfide in the root zone while potentially transferring fixed C and N to the host plant.
Video Abstract
Background
Salt marsh ecosystems are structured by intertidal plant communities at the land-sea interface. Salt marshes are mostly distributed outside of the tropics and comprise a global area of ~5.5 Mha, with approximately 30% of its area located in the continental USA [64]. On the US coastlines of the Atlantic Ocean and Gulf of Mexico, salt marshes are dominated by the smooth cordgrass Spartina alterniflora [69]. Salt marsh ecosystems are biogeochemical hotspots characterized by high rates of primary productivity, organic matter mineralization, and nutrient cycling [36, 43, 48]. As a consequence of their high biological activity, S. alterniflora-dominated salt marshes provide a broad range of ecosystem services to local and global human populations [3]. Ecosystem services provided by salt marshes include estuarine water purification, coastal protection from storm surges, sediment erosion control, maintenance of fisheries, carbon sequestration, and much more [3, 35].
At the local scale, bottom-up control of S. alterniflora primary productivity has been associated with nitrogen (N) uptake kinetics [68]. Low sediment redox potential and high sulfide concentration have been shown to reduce S. alterniflora root energy status, decreasing the plant’s available energy for N uptake [6, 44, 67, 70]. Thus, naturally occurring gradients of S. alterniflora primary productivity are usually found as a function of the plant’s distance to large tidal creeks [96]. Sediments at closer proximity to large tidal creeks are flushed more frequently, supplying oxygen, exchanging porewater nutrients, and oxidizing toxic metabolic products such as sulfide. Conversely, areas in the interior of the marsh tend to be stagnant, accumulating chemically reduced and toxic compounds in their interstitial porewater. The microbial mediation of major biogeochemical cycles along this natural gradient in aboveground S. alterniflora biomass has been extensively studied [18, 39, 47, 48, 73, 93]. However, the relationship between root-microbial interactions and S. alterniflora primary productivity has not been characterized in detail.
Biogeochemical evidence points to tightly coupled interactions between S. alterniflora and microbial activity in the root zone, which facilitate the rapid exchange of electron (e−) acceptors (O2, NO3−, Fe3+) and donors (e.g., rhizodeposits, reduced sulfur compounds). For example, reduced sulfur compounds such as pyrite store high amounts of chemically reduced energy, and their oxidation has long been hypothesized to be an important process limiting the energy flow of salt marsh ecosystems [36]. Part of this energy has been speculated to be used to enhance plant growth [36, 71], similar to the well-known symbiotic relationship between invertebrates and autotrophic sulfur-oxidizing bacteria in marine ecosystems [19]. However, the S. alterniflora root-associated microbiome remains largely unexplored.
Most previous studies investigating the S. alterniflora root microbiome focused on understanding the factors influencing the activity and taxonomic diversity of root-associated nitrogen-fixing bacteria or diazotrophs [7, 16, 26, 57, 100]. A few reports of other functional guilds have shown that chemolithoautotrophs conserving energy through S, Fe, and ammonium oxidation are also enriched in the S. alterniflora root zone when compared to bulk sediment [45, 92, 103]. A drawback of these previous studies is that the majority did not thoroughly separate the root-associated compartment from the surrounding rhizospheric sediment (e.g., [45, 92, 104]). Thus, the ecology of the closely associated S. alterniflora root microbiome and its interaction with the plant host still represents an important knowledge gap to be addressed. In other plants, root microbial communities have been shown to be key players in improving plant resistance to biotic and abiotic stress, outcompeting soil-borne pathogens, modulating plant development, and transferring nutrients for plant uptake [55, 99, 102].
To gain a predictive understanding of the beneficial interactions between a host plant and its associated root microbiome, the ecology and potential physiology of its core microbiome must be investigated. A host’s core microbiome is composed of microbial taxa consistently found associated with host individuals and hypothesized to perform key functions in healthy host-microbiome systems (Shade and Handelsman, 2011). The S. alterniflora core root microbiome has yet to be defined. Understanding of the S. alterniflora root microbiome could pave the way to harnessing plant-microbe interactions for the adaptive management and restoration of salt marsh habitats.
Thus, this study sought to elucidate the plant-microbe interactions linked to S. alterniflora primary productivity over the course of 2 years at 2 sites in GA, USA. The objectives of the study were to 1) evaluate the taxa that constitute the S. alterniflora rhizosphere and root core microbiome in GA, USA; 2) characterize the potential metabolism, physiology, and ecology of the prokaryotic taxa enriched at closer proximities to S. alterniflora roots along primary productivity gradients; and 3) propose a mechanistic understanding of the relationship between S. alterniflora and its root-associated prokaryotic community.
Results
Primary productivity gradient
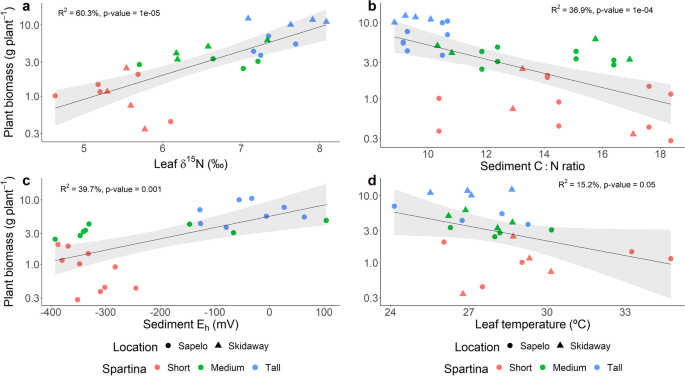
Eight transects along a S. alterniflora primary productivity gradient were studied in two barrier islands in the state of Georgia, USA. A total of 24 sampling points were established and sampled during the years 2018 and 2019 in the Georgia Coastal Ecosystem-Long Term Ecological Research (GCE-LTER) site 6 at Sapelo Island (Lat 31.389° N, Long 81.277° W) and the Saltmarsh Ecosystem Research Facility (SERF) adjacent to the Skidaway Institute of Oceanography on Skidaway Island (Lat 31.975° N, Long 81.030° W) (Fig. S1). S. alterniflora shoot height and biomass revealed a pronounced primary productivity gradient with ranges observed within sampled points from 16.5 to 128.4 cm and 1.4 to 1769.8 g m−2, respectively. S. alterniflora plants were operationally classified in three phenotypes based on shoot height: short (< 50 cm), medium (50–80 cm), and tall (> 80 cm). Shoot biomass averaged 149.4 g m−2, 307.8 g m−2, and 958.7 g m−2 in the short, medium, and tall phenotype zones, respectively. Although shoot biomass was not closely associated with changes in leaf N concentration and total inorganic N in interstitial porewater, a strong relationship with leaf δ15N and sediment C:N ratio was observed (Fig. 1a, b, and Figs. S2, S3, and S4). Higher shoot biomass was also associated with zones in the marsh with elevated sediment redox potential (Eh), which was evidenced not only by direct redox measurements, but also by higher concentrations of interstitial Fe3+, and NO3− in the zones dominated by the tall S. alterniflora phenotype (Fig. 1c, Fig. S3). Conversely, in zones dominated by the short and medium phenotypes, concentrations of porewater ∑S2− were elevated, reaching up to 1.5 mM (Fig. S3). Shoot biomass also showed a negative relationship with leaf temperature, a proxy for leaf stomatal conductance ([79]; Fig. 1d).
Statistical comparisons by linear regression analysis of average plant biomass and leaf natural 15N abundance (δ15N) (a), sediment C:N ratio (b), sediment redox potential (Eh) (c), and leaf temperature (d). Plant biomass was calculated as the average of 10 individuals per sampling point. Each leaf δ15N, sediment C:N ratio, redox potential, and leaf temperature value represents the average of 3, 1, 3, and 5 replicates, respectively
Rates of extracellular enzyme activity for enzymes that catalyze the catabolism of organic C, N, and P compounds showed a strong relationship to S. alterniflora primary production. Rates of extracellular β-glucosidase (C), chitinase (C and N), and phosphatase (P) activity in homogenized sediment slurries were consistently higher in zones with greater S. alterniflora shoot biomass at both Sapelo and Skidaway Island (Fig. 2).
Statistical comparisons by linear regression analysis of enzyme activity rates and plant biomass assessed at Skidaway Island (a) (c) (e) and Sapelo Island (b) (d) (f). For each sample, rates were calculated from 8 time point measurements. Plant biomass represents the average of 10 individuals per sampling point
Microbiome diversity
Prokaryotic diversity and abundance were investigated across the S. alterniflora biomass gradient in three compartments: bulk sediment, rhizosphere, and root. The root compartment was recovered by sonication in an epiphyte removal buffer, thus likely containing mostly endosphere with residual rhizoplane microbial communities [81]. A total of 32,740 unique amplicon sequence variants (ASVs) were inferred using DADA2 v.1.10 [9]. After quality filtering, 10,068,980 high-quality small subunit ribosomal RNA (SSU rRNA) sequence reads with a median depth of 49,619 reads per sample were used for microbiome analysis (further details in the “Materials and methods” section). Prokaryotic communities associated with the tall S. alterniflora bulk and rhizosphere sediment were more diverse and abundant when compared to those of the short phenotype (Fig. 3a, b). In the root compartment, alpha diversity and prokaryotic abundance were highest in the short phenotype (Fig. 3a, b). The root compartment showed a significant decline in alpha diversity in all plant phenotypes driven by a decrease in both richness and evenness when compared to their bulk and rhizospheric counterparts (Fig. 3a, c). Mean richness ± 95% confidence interval (CI95%) across microbiome compartments was 922 ± 40, 981 ± 33, and 695 ± 40 observed ASVs for bulk sediment, rhizosphere, and root prokaryotic communities, respectively. Decreased evenness in the root compartment was evidenced by the presence of highly dominant ASVs (Fig. 3c). Despite the decrease in prokaryotic diversity in the root, abundance remained high, in the range of 107 SSU rRNA gene copies g−1fresh root in both tall and short S. alterniflora phenotypes (Fig. 3b).
Diversity and abundance of the S. alterniflora microbiome. Boxplots of the Shannon diversity index (a) and prokaryotic abundance determined by qPCR of SSU rRNA genes (b) per microbiome compartment and S. alterniflora phenotype. Evenness across plant compartments assessed by a cumulative rank-abundance plot (c). Non-metric multidimensional scaling (nMDS) ordination of the Bray-Curtis dissimilarity matrix across all collected samples with colors representing microbiome compartment (d) and S. alterniflora phenotype (e). nMDS stress 0.10
S. alterniflora phenotype and microbiome compartment were the most significant deterministic forces controlling prokaryotic community assembly in GA salt marshes (Fig. 3d, e, Table 1). Permutational multivariate analysis of variance (PERMANOVA) analysis using the Bray-Curtis dissimilarity index showed that S. alterniflora phenotype explained less species exchange in the root when compared to the other two compartments (Table 1).
Phylogenetic community structure was assessed with the nearest taxon index (NTI) and beta nearest taxon index (βNTI) for within and between prokaryotic communities, respectively [86, 87]. An NTI value greater than 2 indicates greater phylogenetic clustering within a community than expected by chance. All bulk and rhizospheric prokaryotic communities had an NTI greater than 2, while 91% of the root prokaryotic communities met this threshold. NTI values decreased in closer proximity to roots (Fig. S5). Average ± CI95% NTI values per microbiome compartment were 7.0 ± 0.3, 5.6 ± 0.2, and 3.8 ± 0.3 for prokaryotic communities from bulk sediment, rhizosphere, and root compartments, respectively. In order to assess phylogenetic turnover between similar environments in the investigated salt marshes, βNTI values between samples from the same microbiome compartment, S. alterniflora phenotype, year, and location were calculated. An βNTI value lower than −2 indicates less phylogenetic turnover between samples than expected by chance. Pairwise comparison between bulk sediment, rhizosphere, and root prokaryotic communities revealed that 92.2%, 92.6%, and 77.8% βNTI values were below −2, respectively. Similar to NTI analysis, a trend of lower phylogenetic relatedness was observed closer to the root (Fig. S5). Average ± CI95% βNTI was −5.2 ± 0.3, −4.6 ± 0.3, and −3.1 ± 0.2 in bulk sediment, rhizosphere, and root pairwise comparisons, respectively. The microbiome compartment effect on NTI and βNTI was consistent in all S. alterniflora phenotypes.
S. alterniflora root-associated prokaryotic community composition
Overall, at the phylum level, prokaryotic communities were predominated by ASVs from the Proteobacteria (46.7%), Chloroflexi (15.2%), Bacteroidetes (8.4), Epsilonbacteraeota (3.7%), Spirochaetes (3.6%), and Acidobacteria (3.5%) phyla (Fig. S6). At higher S. alterniflora biomass, an increase in the relative abundance of Proteobacteria ASVs and a decline in the relative abundance of Chloroflexi and Spirochaetes ASVs was observed (Fig. S6). At increasing proximity from the root, the relative abundance of Proteobacteria, Spirochaetes, and Epsilonbacteraeota increased while Acidobacteria and Bacteroidetes decreased (Fig. S6). Prokaryotic taxa with the potential to catalyze redox reactions in the S, Fe, and N cycles were investigated in greater detail due to their known significance in salt marsh ecosystem functioning. Putative function was inferred based on homology at the genus level with described prokaryotic species (Table S1). Prokaryotes putatively capable of nitrification (a.k.a. nitrifiers) exhibited higher relative abundance in areas colonized by the tall S. alterniflora phenotype, in comparison to areas occupied by the short and medium phenotypes (Fig. 4a). Dominant nitrifiers in the studied system included members of the bacterial genera Candidatus Nitrotoga and Nitrospira, as well as the archaeal genus Candidatus Nitrosopumilus (Fig. 4a). Additionally, a significant enrichment in taxa potentially involved in the Fe and S cycles was detected in the plant root relative to the bulk sediment (Fig. 4b, c, d). The putative Fe oxidizer of the Zetaproteobacteria, Mariprofundus sp., showed high relative abundance in the roots of the tall S. alterniflora phenotype, while Acidihalobacter of the Gammaproteobacteria was the predominant Fe oxidizer in the roots of the short phenotype (Fig. 4b). Putative autotrophic endosymbionts capable of S oxidation from the Candidatus Thiodiazotropha genus and Thiomicrospirales order preferably colonized the roots of S. alterniflora regardless of plant phenotype (Fig. 4c). Sulfur oxidizers from the Sulfurovum genus preferentially colonized the areas dominated by the short S. alterniflora phenotype in all compartments (Fig. 4c). Putative sulfate reducers of the Desulfobacterales order: Desulfatitalea, Desulfopila, and Desulfosarcina genera were enriched at closer proximities to the S. alterniflora root (Fig. 4d).
Based on differential abundance analysis performed in DESeq2, many ASVs were shown to be significantly enriched in the root compartment. Interestingly, many of the enriched taxa appear to be capable of N fixation, including putative sulfur oxidizers Candidatus Thiodiazotropha, Desulfovibrio, and Arcobacter, S/sulfate reducers Sulfurospirillum, Desulfatitalea, Novosphingobium, Azoarcus, and Celerinatantimonas bacteria (Fig. S7a). Taxa significantly enriched in the tall S. alterniflora phenotype comprised nitrifiers from the Nitrospira and Candidatus Nitrosopumilus genera, putative metal (Fe and Mn) reducer Georgfuchsia, and a diverse set of aerobic or facultative anaerobic chemoheterotrophs (Fig. S7b).
S. alterniflora core microbiome
Taxa consistently found in independent host-microbiome samples have been suggested to perform key functions in healthy host-microbiome interactions (Shade and Handelsman, 2011), and the set of persistent taxa have been defined as the host’s core microbiome. For this study, an ASV prevalence threshold was operationally defined by plotting the relative abundance and richness of the rhizosphere and root core microbiomes at 10% intervals from 0 to 100% ASV prevalence cutoffs (Fig. S8). A conservative prevalence cutoff of 60% was determined by visually inspecting a threshold in which richness remained stable at increasing cutoff values (Fig. S8). The S. alterniflora core root microbiome was composed of only 38 out of 14,505 ASVs and 54 out of 19,435 ASVs in the root and rhizosphere, respectively. However, in both cases, the core microbiome comprised approximately 20% relative abundance of the total prokaryotic community (Fig. S8). Both the root and the rhizosphere core microbiomes were dominated by taxa with inferred metabolic potential for S redox reactions (Fig. 5). The S. alterniflora root core microbiome was comprised of putative autotrophic S oxidizers of the Sulfurovum and Candidatus Thiodiazotropha genera (Fig. 5), while sulfate reducers were represented by ASVs from the Desulfatiglans, Desulfocarbo, Desulfatitalea, Desulfobulbus, Desulfopila, Desulfosarcina, SEEP-SRB1, and Sva0081 genera (Fig. 5). Core root taxa with diazotrophic potential included ASVs from the Candidatus Thiodiazotropha, Desulfatitalea, Desulfobulbus, and Spirochaeta genera (Fig. 5). In the rhizosphere, the proportion of ASVs with unknown classification at the genus level according to the SILVA database (release 132) comprised up to ~60% relative abundance of the core microbiome. Core rhizosphere ASVs with the putative capacity for S oxidation included members of the Sulfurovum and Thioalkalispira genera (Fig. 5). Similar to the root core microbiome, nearly half of the identified taxa at the genus level in the core rhizosphere presented sulfate reduction capability, such as ASVs from the Desulfatiglans, Desulfocarbo, Desulfatitalea, Desulfosarcina, SEEP-SRB1, and Sva0081 genera (Fig. 5). Putative nitrifiers from the Candidatus Nitrosopumilus genus were members of the rhizosphere core microbiome (Fig. 5). Finally, taxa with N fixation capability in the rhizosphere core microbiome included putative sulfur oxidizers from the Thioalkalispira genus, sulfate reducers from the Desulfatitalea genus, and bacterium from the Spirochaeta genus (Fig. 5).
Prokaryotic identity and relative abundance of the S. alterniflora rhizosphere and root core microbiome. Phylogenetic characterization was conducted using an approximately maximum-likelihood model of the 72 ASVs comprising the S. alterniflora core rhizosphere and root microbiome. Taxonomic information at the genus level is provided for all ASVs. When the taxonomic assignment was unknown at the genus level, the “Unk” prefix was used before the highest resolution taxonomic level assigned
Discussion
Biogeochemical processes linked to S. alterniflora primary productivity at the local scale
S. alterniflora primary productivity is strongly linked to N uptake kinetics in field and lab studies [37, 66, 68]. At the local scale, reduced, anoxic, and sulfidic root conditions were shown to lead to a decline in root energy status, affecting ammonium uptake kinetics [44, 68]. In parallel, elevated salinity has been associated with a decrease in stomatal conductance and photosynthetic activity, along with an increase in dark respiration [29, 38]. Our observations corroborate these past results showing that S. alterniflora primary productivity is hampered at reduced sediment Eh and under highly sulfidic conditions, when the plants experience limited leaf gas exchange. Results from this study also support our previous research which revealed the dynamic interplay between the growth/physiology of macrophyte plants and macrofaunal bioturbation [30, 48], with crab burrow density shown to directly correlate with aboveground plant biomass and sediment redox potential (Fig. S2).
Significant differences in the stable N isotope composition of sediment and S. alterniflora leaves provide evidence that N sources and dynamics are distinct along the studied S. alterniflora primary productivity gradient [13]. We argue that elevated leaf and sediment δ15N in the tall S. alterniflora phenotype is the result of (i) greater nitrogen loss by coupled nitrification-denitrification and (ii) differences in the source of N input. Denitrification in Georgia salt marshes is limited and tightly coupled to prokaryotic nitrification, which discriminates against 15N [13, 18]. Prokaryotes capable of nitrification show higher relative abundance in sediments dominated by the tall S. alterniflora phenotype (Fig. 4a), and their activity is known to be inhibited by sulfide toxicity [18, 41]. Porewater sulfide concentration in the short and medium S. alterniflora phenotype was an order of magnitude higher than in the tall phenotype. A second explanation for δ15N enrichment in the tall S. alterniflora phenotype is the greater source of planktonic N at closer proximities to the tidal creek. Planktonic tissue has a δ15N signature of 8.6 ± 1.0‰ [76], which is more similar to the leaf δ15N measured in the tall S. alterniflora (7.6 ± 0.3‰) when compared to the short phenotype (5.5 ± 0.4‰) (Fig. S4). A larger discrepancy in δ13C between sediment and leaf tissue in the tall S. alterniflora phenotype, as well as a lower sediment C:N ratio, supports our interpretation of a reduced relative contribution of vascular plant material to organic matter diagenesis (Fig. S4, Fig. 1b, [21, 28]). The average ± CI95% δ13C signature in sediments from the tall S. alterniflora phenotype (−20.3 ± 0.4 ‰) more closely resembles that of reported phytoplankton, which enters the marsh via tidal creeks (−17 to −24‰, [22]; −21.3 ± 1.1‰, [76]; −20.16‰, [15]). The C:N ratio is considered as a proxy for soil organic matter reactivity, and a lower C:N ratio indicates a greater potential for rapid biodegradation and N mineralization [40]. In agreement with this interpretation, sediments with low C:N ratios from the tall S. alterniflora zone contained higher prokaryotic biomass, and higher rates of extracellular enzyme activities involved in the C, N, and P cycles. We propose that plant primary productivity is enhanced in the tall S. alterniflora zone in part due to more rapid microbial mineralization of higher-quality sediment organic matter of planktonic origin, with released inorganic nutrients then made available for plant uptake.
Assembly of the S. alterniflora root microbiome
Previous studies have reported contradictory results with regard to the relationship between the diversity of the S. alterniflora microbiome, plant productivity, and proximity to the root [45, 54, 104]. Our finding of higher prokaryotic alpha diversity in bulk and rhizospheric sediment associated with the tall S. alterniflora phenotype is consistent with previous findings from Skidaway Island, GA [45]. However, Zogg et al. [104] and Lin et al. [54] did not observe significant differences in prokaryotic alpha diversity between the tall and short S. alterniflora phenotypes in bulk and rhizospheric sediments from New England and Guangdong province in China, respectively. Contrasting results could be due to limitations in methodology and experimental design in previous work. Sequencing platforms continue to evolve, enabling higher sequence coverage at lower cost, and our sampling effort was approximately an order of magnitude more intensive than previous studies characterizing the S. alterniflora root microbiome, including our own past work [45, 54, 104]. Moreover, recently developed in silico technology to infer ASVs instead of clustering sequences into operational taxonomic units (OTUs) could also impact the estimation of alpha diversity metrics.
Few studies have investigated microbial diversity in wetland plant roots. Nonetheless, consistent with our results, a single report from S. alterniflora and studies from other wetland/estuarine plants show a decline in diversity in the root compared to the bulk and rhizosphere compartments [20, 34, 61]. Prokaryotic abundance in the endosphere has been previously estimated in the 104 to 108 cells g−1 range across an array of plant species [8]. Taking into consideration that many of these previous estimates employed cultivation-based methods, and prokaryotic genomes often contain multiple SSU rRNA operons, our observation of prokaryotic abundance (107 SSU rRNA gene copies g−1) would be placed at the high-end of that range. A marked decrease in prokaryotic alpha diversity with high abundances is an indication that the S. alterniflora root compartment is enriched in dominant and highly active species taking advantage of labile carbon sources, reduced inorganic compounds, and the oxidized environment found in the S. alterniflora root [11, 59]. Increased relative abundances of S and Fe chemolithotrophs, and aerobic and facultative anaerobic chemoorganotrophs at closer proximities to the root further support this interpretation.
Microbial community assembly in the root endosphere has been proposed as a two-step colonization process [8]. The first step is driven by microbial proliferation in the rhizosphere by species capable of utilizing plant-released substrates, while the second is a fine-tuning step in the rhizoplane, where selection by the plant’s genotype-dependent immune system takes place [8]. Endospheric microbial species have co-evolved to evade the plant immune system by secreting effector proteins that mimic plant proteins [95]. In S. alterniflora, a decrease in prokaryotic richness in the root compartment and the fact that plant phenotype was the most important deterministic factor assembling the root prokaryotic community suggest that plant selection is an important process in community assembly. However, community assembly is a result of co-occurring deterministic and stochastic processes [17, 87]. Generally, environmental filtering has been shown to be the main deterministic process assembling microbial communities spatially, as evidenced by phylogenetic clustering [23, 86]. Nevertheless, in our study, environmental filtering was relaxed at closer proximity to the root, a microenvironment characterized by an abundance of high-quality e− donors and acceptors. Most likely, increased competition, the dominance of fast-growing bacteria filling a resource-rich niche, and historical contingency (i.e., first prokaryotic species to colonize the root successfully outcompete other taxa) are co-occurring ecological processes reducing the relative importance of environmental filtering in the root zone [24, 31, 94]. Increased species dominance, decreased richness, and increased phylogenetic dispersion with high prokaryotic abundances in the S. alterniflora root support this hypothesis.
Characterization of the S. alterniflora core microbiome and potential plant-microbe interactions driving primary productivity
Our results indicate that putative sulfate-reducing and sulfur-oxidizing prokaryotes comprise a large proportion of the S. alterniflora root and rhizosphere core microbiomes. Sulfate-reducing communities are comprised of metabolically versatile populations capable of utilizing a broad range of C substrates, including plant-derived substrates [2]. The most dominant sulfate reducer genus in the S. alterniflora core root and rhizosphere, Desulfatitalea, mainly utilizes short-chain fatty acids as an electron donor and C source [32]. However, other sulfate-reducing members of the root core microbiome, such as Desulfatiglans and Desulfocarbo, have been shown to oxidize plant-derived aromatic compounds (e.g., lignin) [1, 90]. In salt marsh ecosystems, biogeochemical data indicates that the C and S cycles are tightly coupled, with S. alterniflora photosynthetic activity fueling the activity and C utilization of sulfate reducers in the rhizosphere [39, 49, 85]. Similarly, sulfide oxidizers thrive in the S. alterniflora root zone, especially in the short phenotype, where sulfide concentration is elevated ([45, 92]; this study). In our study, ASVs from the Sulfurovum genus were enriched in all compartments of the short S. alterniflora phenotype. This genus has been described as highly versatile and diverse, allowing for efficient niche partitioning in highly dynamic sulfidic and oxic environments [65, 72]. The co-occurrence of prokaryotic ASVs associated with S anaerobic and aerobic metabolisms in the S. alterniflora rhizosphere and root, and highly dynamic O2 concentrations at the microscale [46] support the interpretation of a rapid and cryptic S cycle at close proximity to, or even inside, the root tissue. Rapid and coupled cycling of C and S are crucial to the replenishment of nutrients and electron acceptors in the S. alterniflora root zone to support high microbial and plant activity. Rapid organic matter mineralization is especially relevant to effectively recycle N, most often the limiting nutrient for plant productivity in the salt marsh (the “Biogeochemical processes linked to S. alterniflora primary productivity at the local scale” section).
Previous studies in marshes of the southeastern US show that plant photosynthetic activity and rhizodeposition stimulate N fixation by root-associated sulfate reducers [26, 57, 100]. Consistently, prokaryotic species from the Desulfobulbus, Desulfatitalea, Desulfovibrio, and Sulfurospirillum genera, either significantly enriched or members of the S. alterniflora root core microbiome, have been shown to couple sulfate or sulfur respiration to N fixation [33, 91]. Species from the root and rhizosphere core microbiome genera Candidatus Thiodiazotropha and Thioalkalispira have the metabolic potential to couple S oxidation with C and N fixation [4, 75]. Intriguingly, Candidatus Thiodiazotropha is a recently described genus of endosymbionts discovered in the gills of lucinid bivalves [75]. Lucinid bivalves are not generally found in salt marshes, but they have been associated with reduced plant sulfide stress in seagrass meadows and mangroves [25, 52, 53]. In these ecosystems, a tripartite symbiotic relationship occurs, whereby bivalves provide O2 to endosymbiont sulfide oxidizers that detoxify the plant’s environment from sulfide, a known phytotoxin [25]. Recent studies revealed that Candidatus Thiodiazotropha is also an important and prevalent member of the seagrass root microbiome, suggesting that these chemolithoautotrophic S oxidizers form a direct association with subtidal marine plant species without the need of a lucinid bivalve partner [14, 62]. Our study confirms this finding and expands the distribution range of Candidatus Thiodiazotropha to the roots of intertidal, estuarine plant species.
Although sulfide is generally considered as a potent phytotoxin, moderately sulfidic conditions (< 1 mM) have been shown to actually stimulate S. alterniflora growth in a controlled laboratory experiment [71]. Energy conservation from sulfide oxidation in the root tissue was speculated to be the driver of increased plant primary production [68]. Furthermore, sulfide oxidation to sulfate has been demonstrated inside S. alterniflora root tissues using isotope tracers [11, 50]. However, it is still not clear what process, biological or chemical, dominates sulfide oxidation inside S. alterniflora roots. We propose that S. alterniflora shares a symbiotic relationship with S oxidizers in both the rhizosphere and root compartments. Sulfur oxidation may be mediated by not only Candidatus Thiodiazotropha bacteria, but also members of the Sulfurovum and Thioalkalispira genera or endosymbionts from the Thiomicrospirales order. Previously studied microbial species from the Sulfurovum genus and endosymbionts from the Thiomicrospirales order have been demonstrated to fix C; whereas members from Desulfovibrio, Thioalkalispira, and Candidatus Thiodiazotropha genera have been shown to perform both C and N fixation [4, 75, 89, 91]. Moreover, Crump et al. [14] studying the root microbiome of seagrass Zostera spp. found high transcript levels of N fixing and sulfur-oxidizing genes from Gammaproteobacteria species, including endosymbionts of marine invertebrates from the Sedimenticolaceae family, which includes the Candidatus Thiodiazotropha genus. Given that the S. alterniflora root zone is enriched in reduced S and its growth is limited by N uptake, we suggest that diazotrophy coupled to sulfide oxidation may be a key process that was previously overlooked. However, direct measurements of N and C fixation, and sulfur oxidation in the roots of S. alterniflora, their rates and controls, along with their relative contribution to plant growth remain unclear and require further research. Given that most studies, including this study, have inferred the coupling of S oxidation and N fixation based on gene homology and/or taxonomic placement, this interpretation should be treated with caution.
Conclusions
We studied a gradient in S. alterniflora productivity to characterize the ecology and physiology of the S. alterniflora root-associated microbiome and its potential role in shaping plant physiological performance. In sediments from the tall S. alterniflora phenotype, higher prokaryotic biomass and more rapid microbial mineralization of organic matter were linked to greater inorganic nutrient replenishment for plant uptake. Prokaryotic communities from bulk and rhizospheric sediment associated with the tall S. alterniflora phenotype contained the highest alpha diversity, while a decline in diversity was observed in the root in comparison to the bulk and rhizosphere sediment compartments in all S. alterniflora phenotypes. A marked decrease in prokaryotic alpha diversity with high abundances and increased phylogenetic dispersion was observed in the S. alterniflora root compartment. Thus, we propose that the S. alterniflora root microbiome is dominated by highly active and competitive species taking advantage of available carbon substrates in the oxidized root zone. The high relative abundance of prokaryotic ASVs with putative S oxidation and sulfate reduction capability in the S. alterniflora rhizosphere and root suggests a rapid S cycle at close proximity to, or even inside, the root tissue. Moreover, both functional guilds were overrepresented in the S. alterniflora rhizosphere and root core microbiome. Rapid recycling of S is crucial for organic matter mineralization in anoxic marsh sediments. Thus, we propose that S. alterniflora shares a symbiotic relationship with S oxidizing bacteria in both the rhizosphere and root compartments. Sulfur oxidizers may benefit S. alterniflora plants not only by removing potentially toxic sulfide from the root zone, but also by coupling S oxidation with N and/or C fixation. The contribution to plant growth of each of these microbial processes represents a knowledge gap that warrants further research.
Materials and methods
Sampling design and general site description
The study was carried out in two salt marshes for which long-term data is available in the state of Georgia, USA: (i) the Georgia Coastal Ecosystem-Long Term Ecological Research (GCE-LTER) site 6 at Sapelo Island (Lat 31.389° N, Long 81.277° W) and (ii) the Saltmarsh Ecosystem Research Facility (SERF) adjacent to the Skidaway Institute of Oceanography on Skidaway Island (Lat 31.975° N, Long 81.030° W) (Fig. S1). The GCE-LTER site was sampled twice, during July 2018 and 2019, while the SERF site was sampled once in July 2019. Four ~100-m transects adjacent to large tidal creeks with two to four sampling points along S. alterniflora primary productivity gradients were sampled at each site (total: 24 sampling points, Fig. S1).
At each sampling point along the transects, a 50 cm × 50 cm quadrat was established to measure the density of marsh periwinkle snails (Littoraria irrorata) and fiddler crab (Uca pugnax) burrows. In situ sediment pH and redox potential (Eh) were measured in triplicate at two sediment depths (2.5 and 7.5 cm) with a hand-held pH/ORP meter during the two sampling events at Sapelo Island (HI-98121 tester, Hanna instruments, Woonsocket, RI, USA).
S. alterniflora ecophysiology and elemental analysis
S. alterniflora shoot density was quantified at every sampling point in 50 cm × 50 cm quadrats, and shoot height measured for 10 plants per sampling point. S. alterniflora plants were operationally classified in three phenotypes based on shoot height: short (< 50 cm), medium (50 – 80 cm), and tall (> 80 cm). S. alterniflora shoot biomass was estimated by allometry, with an equation calibrated at Sapelo Island (Table S2, [101]). During the 2019 sampling events, leaf C and N concentrations and 13C and 15N isotopic natural abundance were determined for 3 plants per sampling point with elemental and isotope analyses conducted at the University of Georgia – Center for Applied Isotope Studies (CAIS https://cais.uga.edu/). Leaf elemental analysis was performed by the micro-Dumas method, while isotopic natural abundance was measured by isotope ratio mass spectrometry. 13C natural abundance was expressed as the per mille (‰) deviation from the Pee Dee Belemnite standard (PDB) 13C:12C ratio (δ13C), while 15N natural abundance expressed as the ‰ deviation from the N2 atmospheric 15N:14N ratio (δ15N). Leaf temperature, a proxy for stomatal conductance [79], was measured between 11:00 and 12:00 utilizing a Fluke-62 MAX+ infrared thermometer (Fluke Co. USA, Everett, WA). All leaf ecophysiological measurements were performed in young, expanded, and sun-exposed leaves.
Porewater and sediment sampling and chemical analysis
Rhizon samplers with 0.15-μm pore size filters (model CSS, Rhizosphere Research Products, Wageningen, The Netherlands, https://www.rhizosphere.com/rhizons) were used to extract sediment porewater at 2.5- and 7.5-cm depth from every sampling point. For each porewater sample, a 2-ml subsample was frozen at −20°C for salinity, nitrate, ammonium, and phosphate concentration analysis; a 2-ml subsample was immediately acidified with 20 μl 12N HCl for Fe2+ and Fe3+ concentration analysis; and a 100-μl subsample was immediately fixed into 1 ml zinc acetate 2% (w/v) solution for sulfide concentration analysis [39, 48].
Porewater concentrations of nitrate, ammonium, phosphate, sulfide, Fe3+, and Fe2+ were quantified based on spectrophotometric methods as previously described [12, 27, 84, 88, 98]. Porewater chloride concentration was determined by HPLC with ultraviolet detection as described by Beckler et al. [5]. Salinity was calculated based on porewater chloride concentration.
Sediment samples were collected at all sampling points from two depth intervals, 0–5 cm and 5–10 cm. An approximately 30-g sediment subsample was oven-dried at 60°C for 72 h. The oven-dried sample was homogenized using a PowerGen high-throughput homogenizer (Fisherbrand, Pittsburgh, PA) and sent to the University of Georgia CAIS (https://cais.uga.edu/) for organic C and N concentration and 13C and 15N isotopic natural abundance analyses. Sediment organic C and 13C isotopic natural abundance was analyzed in acid-fumigated samples [80].
Samples collected for microbial community analysis were flash-frozen in an ethanol and dry ice bath and stored at −80°C until nucleic acid extraction. Sediment samples collected for enzymatic rates were kept at 4 °C and analyzed within 4 h after sampling.
Extracellular enzyme activity
Rates of extracellular enzymatic activity (β-glucosidase, phosphatase, and chitinase) were measured in 2019 in Sapelo and Skidaway Island. Technical duplicates of 0–10-cm-deep sediment samples were collected for each sampling point. Rates were analyzed in a homogenized sediment slurry using fluorescent 4-methylumbelliferone (MUF)-linked substrates (Table S3). Slurry preparation consisted of mixing wet sediment with 50 mM Tris buffer (pH 7) in a 1:2 (w/v) ratio. The slurry was homogenized with a stomacher homogenizer (model 400; Seward Medical, London, England) at 200 rpm for 30 s in ~1-mm filter bags. Sediment slurry (800 μl) was incubated in the dark with 200 μl of fluorescent-linked substrate (initial substrate concentration 40 μM). Product accumulation was measured at 0, 0.5, 1, 1.5, 3, 4, 6, and 8 h after the start of the incubation based on fluorescence intensity (excitation 355 nm, emission 460 nm) using a microplate fluorescence reader (SpectraMax M2, Molecular Devices, San Jose, CA). Enzymatic rates were calculated by fitting a linear regression and reported in μmol kgwet sediment−1 h−1.
Plant sampling, compartment fractionation, and molecular biology
S. alterniflora roots were sampled at two depths (0–5 and 5–10 cm). Sediment loosely attached to the root was immediately washed two times with creek water in the field. Roots with remaining rhizospheric sediment were flash-frozen in an ethanol-dry ice bath and stored at −80°C until analysis.
Plant compartment separation
Separation of the root and rhizosphere compartments was performed by sonication in an epiphyte removal buffer (0.1% (v/v) Triton X-100 in 50 mM potassium phosphate buffer) [81]. Sonication was performed at 4°C for 10 min with pulses of 160W for 30 s interspersed with 30-s pauses. Sediment detached from the roots was centrifuged and considered to be the rhizosphere compartment, while the sonicated roots were considered as the root compartment. The root compartment was washed with PBS buffer (pH 7.4) three consecutive times and ground with liquid N before DNA extraction. Simmons et al. [81] protocol was designed to isolate endospheric DNA; however, since we did not confirm the separation by microscopy, it is possible that our root compartment contained residues of the rhizoplane compartment.
Removal of extracellular DNA
Prior to bulk sediment DNA extraction, extracellular dissolved or sediment-adsorbed DNA was removed according to Lever et al. [51]. Briefly, 2 g of sediment was incubated with 2 ml carbonate dissolution solution (0.43 M sodium acetate, 0.43 M acetic acid, 10 mM EDTA, 100 mM sodium metaphosphate, 3% (w/v) NaCl, pH 4.7) for 1 h while orbital shaking at 300 rpm and room temperature. Afterwards, 16 ml of a 300mM Tris-HCl and 10mM EDTA solution (3% NaCl, pH 10.0) was added into the slurry and incubated for 1 additional hour at the same orbital shaking condition. After centrifugation at 10,000 g for 20 min at room temperature, the pellet was composed by extracellular-DNA free sediment and used for prokaryotic community characterization.
DNA extraction and sequencing library preparation
DNA extraction for all assessed compartments was performed using the DNeasy PowerSoil kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The concentration of extracted DNA was determined with the Qubit HS assay (Invitrogen, Carlsbad, CA). Amplification of the SSU rRNA gene V4 region was performed using the primers 515F (5′-GTGCCAGCMGCCGCGGTAA′) and 806R (5′-GGACTACHVGGGTWTCTAAT′) [10]. Reactions were performed in triplicate of 5-ng DNA template in a solution containing DreamTaq buffer, 0.2 mM dNTPs, 0.5 μM of each primer, 0.75 μM of each mitochondrial (mPNA) and plastid (pPNA) peptide nucleic acid (PNA) clamps, and 1.25 U DreamTaq DNA polymerase as previously described ([45], further details in Table S4). PNA clamps have been shown to reduce plant plastid and mitochondrial DNA amplification in PCR reactions [58]. Triplicate PCR products were pooled together, barcoded with 10-base unique barcodes (Fluidigm Corporation, San Francisco, CA), and sequenced on an Illumina MiSeq2000 platform using a 500-cycle v2 sequencing kit (250 paired-end reads) at the Research Resources Center in the University of Illinois at Chicago. The raw SSU rRNA gene amplicon sequences have been deposited in the BioProject database (http://ncbi.nlm.nih.gov/bioproject) under accessions PRJNA666636.
Quantification of prokaryotic abundance
Prokaryotic abundance was quantified by quantitative polymerase chain reaction (qPCR) of the SSU rRNA gene with general primers in a subset of 24 samples collected in Sapelo Island in 2018 and 2019. The subset comprised superficial (0–5 cm) samples from all three compartments, collected from the four established transects in Sapelo Island. Only samples from the tall and short extreme S. alterniflora phenotypes were included into the analysis. Samples were analyzed in triplicate using the StepOnePlus platform (Applied Biosystems, Foster City, CA, USA) and PowerUp SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). Reactions were performed in a final volume of 20μl using the standard primer set 515F (5′-GTGCCAGCMGCCGCGGTAA′) and 806R (5′-GGACTACHVGGGTWTCTAAT′) specific for the prokaryotic SSU rRNA gene ([10], Table S4). To avoid plant plastid and mitochondrial DNA amplification from rhizosphere and root samples, peptide nucleic acid PCR blockers (PNA clamps, 0.75 μM) were added to all qPCR reactions [58]. Standard calibration was performed from a 10-fold serial dilution (103 to 108 molecules) of standard pGEM-T Easy plasmids (Promega, Madison, WI, USA) containing target sequences from Escherichia coli K12. Specificity of PCR products was confirmed by melting curve analyses. Prokaryotic SSU rRNA gene copy numbers were calculated as gene copy number g−1 of fresh material.
Ecological, phylogenetic, statistical, and bioinformatic analysis
Amplification primers were removed from raw fastq files using Cutadapt v.2.0 [60]. Amplicon sequence variants (ASVs) were inferred from quality-filtered reads utilizing DADA2 v.1.10 [9]. Paired reads were merged, and reads between 251 and 255 bp in length were conserved. Chimeras were removed using the removeBimeraDenovo function from the DADA2 package. Taxonomy was assigned utilizing the Ribosomal Database Project (RDP) Naive Bayesian Classifier [97] against the SILVA SSU rRNA reference alignment (Release 132, [78]). Sequences classified as chloroplast, mitochondrial, and eukaryotic or that did not match any taxonomic phylum were excluded from the dataset. Reads were filtered to remove ASVs that appeared in less than 5% of the samples and/or had less than 10 total counts. A total of 32,740 unique ASVs were aligned to the SILVA SSU rRNA reference alignment (Release 132, [78]) in mothur v.1.43 [82], and an approximately maximum-likelihood tree was constructed using FastTree v.2.1 [77]. Finally, 10,068,980 high-quality SSU rRNA sequence reads with a median depth of 49,619 reads per sample were used for subsequent analysis.
Shannon diversity index was estimated using the phyloseq v.1.26 package [63]. Non-metric multidimensional scaling (nMDS) ordination utilizing the Bray-Curtis dissimilarity distance was performed. Multivariate variation of the Bray-Curtis dissimilarity matrix was partitioned to microbiome compartment (bulk sediment, rhizosphere, and root), S. alterniflora phenotype (tall, medium, and short), depth (0–5 cm and 5–10 cm), location (Sapelo Island and Skidaway Island), and year (2018 and 2019) based on a permutational multivariate analysis of variance (PERMANOVA) analysis with 999 permutations performed in vegan v. 2.5 [74]. PERMANOVA analysis was run for the complete dataset and in subsets per microbiome compartment. Differential abundance analysis was performed to assess genera that were significantly enriched in specific plant compartments, and in zones of the marsh associated with different S. alterniflora phenotypes, using DESeq2 v.1.26 [56].
To evaluate phylogenetic community structure within (alpha) and between (beta) communities, we quantified the nearest taxon index (NTI) and the beta nearest taxon index (βNTI), respectively [86, 87]. NTI and βNTI indices were calculated as the number of standard deviations of the observed mean-nearest-taxon-distance (MNTD) and βMNTD from a null distribution (999 randomizations of all ASV names across phylogenetic tree tips) using the picante package v. 1.8 [42]. For within community analysis, an NTI greater than +2 indicates that coexisting taxa are more closely related than expected by chance (phylogenetic clustering due to environmental filtering), while an NTI less than −2 indicates that coexisting taxa are more distantly related than expected by chance (phylogenetic overdispersion due to greater competition between closely related ASVs) [86]. For βNTI, we assessed pairwise comparisons for samples from the same plant compartment, S. alterniflora phenotype, and sampling event in order to evaluate if phylogenetic structure within communities (NTI) replicated at a greater scale (βNTI between samples occupying the same marsh microenvironment). A βNTI value <−2 or >+2 indicates less or greater than expected phylogenetic turnover between two samples than expected by chance, respectively [86].
The S. alterniflora core root microbiome was investigated. For this study, an ASV prevalence threshold was operationally defined by plotting the relative abundance and richness of the rhizosphere and root core microbiomes at 10% intervals from 0 to 100% ASV prevalence cutoffs (Fig. S8). A conservative prevalence cutoff of 60% was determined by visually inspecting a threshold in which richness remained stable at increasing cutoff values (Fig. S8). Finally, putative nitrifying, S oxidizing, S/sulfate reducing, and Fe oxidizing function was inferred based on homology of ASVs at the genus level with previously described prokaryotic species (Table S1).
Availability of data and materials
Sequence data is available in the BioProject database (http://ncbi.nlm.nih.gov/bioproject) under accessions PRJNA666636. Associated data, metadata, and R script for the bioinformatic pipeline used in this study are available in https://github.com/kostka-lab/Spartina_GA_Core_Microbiome.
Abbreviations
- ASV:
-
Amplicon sequence variant
- C:
-
Carbon
- CAIS:
-
Center for Applied Isotope Studies
- CI95% :
-
95% confidence interval
- e− :
-
Electron
- Eh:
-
Redox potential
- GCE-LTER:
-
Georgia Coastal Ecosystem-Long Term Ecological Research
- MNTD:
-
Mean-nearest-taxon-distance
- mPNA:
-
Mitochondrial peptide nucleic acid
- MUF:
-
4-Methylumbelliferone
- N:
-
Nitrogen
- nMDS:
-
Non-metric multidimensional scaling
- NTI:
-
Nearest taxon index
- OTU:
-
Operational taxonomic unit
- PDB:
-
Pee Dee Belemnite
- PERMANOVA:
-
Permutational multivariate analysis of variance
- PNA:
-
Peptide nucleic acid
- pPNA:
-
Plastid peptide nucleic acid
- qPCR:
-
Quantitative polymerase chain reaction
- RDP:
-
Ribosomal Database Project
- S:
-
Sulfur
- SERF:
-
Saltmarsh Ecosystem Research Facility
- SSU rRNA:
-
Small subunit ribosomal RNA
- βNTI:
-
Beta nearest taxon index
- δ13C:
-
13C abundance expressed as the per mille deviation from the Pee Dee Belemnite standard 13C:12C ratio
- δ15N:
-
15N abundance expressed as the per mille deviation from the N2 atmospheric 15N:14N ratio
References
An TT, Picardal FW. Desulfocarbo indianensis gen. nov., sp. nov., a benzoate-oxidizing, sulfate-reducing bacterium isolated from water extracted from a coal bed. International journal of systematic and evolutionary microbiology. 2014;64:2907–14.
Bahr M, Crump BC, Klepac-Ceraj V, Teske A, Sogin ML, Hobbie JE. Molecular characterization of sulfate-reducing bacteria in a New England salt marsh. Environmental Microbiology. 2005;7:1175–85.
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. The value of estuarine and coastal ecosystem services. Ecological Monographs. 2011;81:169–93.
Barbieri E, Ceccaroli P, Saltarelli R, Guidi C, Potenza L, Basaglia M, et al. New evidence for nitrogen fixation within the Italian white truffle Tuber magnatum. Fungal biology. 2010;114:936–42.
Beckler JS, Nuzzio DB, Taillefert M. Development of single-step liquid chromatography methods with ultraviolet detection for the measurement of inorganic anions in marine waters. Limnology and Oceanography: Methods. 2014;12:563–76.
Bradley PM, Morris JT. Influence of oxygen and sulfide concentration on nitrogen uptake kinetics in Spartina alterniflora. Ecology. 1990;71:282–7.
Brown MM, Friez MJ, Lovell CR. Expression of nifH genes by diazotrophic bacteria in the rhizosphere of short form Spartina alterniflora. FEMS Microbiology Ecology. 2003;43:411–7.
Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaat EVL, Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annual review of plant biology. 2013;64:807–38.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nature methods. 2016;13:581–3.
Caporaso G, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. PNAS. 2011;108:4516–22.
Carlson PR, Forrest J. Uptake of dissolved sulfide by Spartina alterniflora: evidence from natural sulfur isotope abundance ratios. Science. 1982;216:633–5.
Cline JD. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969;14:454–8.
Craine JM, Brookshire ENJ, Cramer MD, Hasselquist NJ, Koba K, Marin-Spiotta E, et al. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant and Soil. 2015;396:1–26.
Crump BC, Wojahn JM, Tomas F, Mueller RS. Metatranscriptomics and amplicon sequencing reveal mutualisms in seagrass microbiomes. Frontiers in Microbiology. 2018;9:388.
Dai J, Sun M-Y, Culp RA, Noakes JE. Changes in chemical and isotopic signatures of plant materials during degradation: implication for assessing various organic inputs in estuarine systems. Geophysical Research Letters. 2005;32:L13608.
Davis DA, Gamble MD, Bagwell CE, Bergholz PW, Lovell CR. Responses of salt marsh plant rhizosphere diazotroph assemblages to changes in marsh elevation, edaphic conditions and plant host species. Microbial ecology. 2011;61:386–98.
Dini-Andreote F, Stegen JC, van Elsas JD, Salles JF. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proceedings of the National Academy of Sciences. 2015;112:E1326–32.
Dollhopf SL, Hyun JH, Smith AC, Adams HJ, O’Brien S, Kostka JE. Quantification of ammonia-oxidizing bacteria and factors controlling nitrification in salt marsh sediments. Applied and Environmental Microbiology. 2005;71:240–6.
Dubilier N, Bergin C, Lott C. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nature Reviews Microbiology. 2008;6:725–40.
Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, et al. Structure, variation, and assembly of the root-associated microbiomes of rice. Proceedings of the National Academy of Sciences. 2015;112:E911–20.
Ember LM, Williams DF, Morris JT. Processes that influence carbon isotope variations in salt marsh sediments. Marine Ecology Progress Series. 1987;36:33–42.
Fogel ML, Sprague EK, Gize AP, Frey RW. Diagenesis of organic matter in Georgia salt marshes. Estuarine, Coastal and Shelf Science. 1989;28:211–30.
Freedman Z, Zak DR. Soil bacterial communities are shaped by temporal and environmental filtering: evidence from a long-term chronosequence. Environmental microbiology. 2015;17:3208–18.
Fukami T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annual Review of Ecology, Evolution, and Systematics. 2015;46:1–23.
Gagnon K, Rinde E, Bengil EG, Carugati L, Christianen MJ, Danovaro R, et al. Facilitating foundation species: the potential for plant–bivalve interactions to improve habitat restoration success. Journal of Applied Ecology. 2020. https://doi.org/10.1111/1365-2664.13605.
Gandy EL, Yoch DC. Relationship between nitrogen-fixing sulfate reducers and fermenters in salt marsh sediments and roots of Spartina alterniflora. Applied and Environmental Microbiology. 1988;54:2031–6.
Garcia-Robledo E, Corzo A, Papaspyrou S. A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Marine Chemistry. 2014;162:30–6.
Gebrehiwet T, Koretsky CM, Krishnamurthy RV. Influence of Spartina and Juncus on saltmarsh sediments. III. Organic geochemistry. Chemical Geology. 2008;255:114–9.
Giurgevich JR, Dunn EL. Seasonal patterns of CO2 and water vapor exchange of the tall and short height forms of Spartina alterniflora Loisel in a Georgia salt marsh. Oecologia. 1979;43:139–56.
Gribsholt B, Kostka JE, Kristensen E. Impact of fiddler crabs and plant roots on sediment biogeochemistry in a Georgia saltmarsh. Marine Ecology Progress Series. 2003;259:237–51.
Hassani MA, Durán P, Hacquard S. Microbial interactions within the plant holobiont. Microbiome. 2018;6:58.
Higashioka Y, Kojima H, Watanabe M, Fukui M. Desulfatitalea tepidiphila gen. nov., sp. nov., a sulfate-reducing bacterium isolated from tidal flat sediment. International journal of systematic and evolutionary microbiology. 2013;63:761–5.
Higashioka Y, Kojima H, Watanabe T, Fukui M. Draft genome sequence of Desulfatitalea tepidiphila S28bFT. Genome announcements. 2015;3(6):e01326–15.
Hong Y, Liao D, Hu A, Wang H, Chen J, Khan S, et al. Diversity of endophytic and rhizoplane bacterial communities associated with exotic Spartina alterniflora and native mangrove using Illumina amplicon sequencing. Canadian journal of microbiology. 2015;61:723–33.
Hopkinson, C.S., Wolanski, E., Brinson, M.M., Cahoon, D.R., and Perillo, G.M.E., 2019. Coastal Wetlands: A Synthesis. In: G.M.E., Perillo, E., Wolanski, D.R., Cahoon, and C.S., Hopkinson. (Eds.) Coastal wetlands, second edition: an integrated and ecosystem approach. Elsevier, pp. 1–75.
Howarth RW. The ecological significance of sulfur in the energy dynamics of salt marsh and coastal marine sediments. Biogeochemistry. 1984;1:5–27.
Howes BL, Dacey JWH, Goehringer DD. Factors controlling the growth form of Spartina alterniflora: feedbacks between above-ground production, sediment oxidation, nitrogen and salinity. Journal of Ecology. 1986;74:881–98.
Hwang, Y-S., Morris, J.T., 1994. Whole-plant gas exchange responses of Spartina alterniflora (Poaceae) to a range of constant and transient salinities. American Journal of Botany, 81:659-665.
Hyun JH, Smith AC, Kostka JE. Relative contributions of sulfate-and iron (III) reduction to organic matter mineralization and process controls in contrasting habitats of the Georgia saltmarsh. Applied Geochemistry. 2007;22:2637–51.
Janssen, B., 1996. Nitrogen mineralization in relation to C:N ratio and decomposability of organic materials. Plant Soil. 181:39–45
Joye SB, Hollibaugh JT. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science. 1995;270:623–5.
Kembel S, Cowan P, Helmus M, Cornwell W, Morlon H, Ackerly D, et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26:1463–4.
Kirwan ML, Guntenspergen GR, Morris JT. Latitudinal trends in Spartina alterniflora productivity and the response of coastal marshes to global change. Global Change Biology. 2009;15:1982–9.
Koch MS, Mendelssohn IA, McKee KL. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnology and Oceanography. 1990;35:399–408.
Kolton, M., Rolando, J.L., Kostka, J.E., 2020. Elucidation of the rhizosphere microbiome linked to Spartina alterniflora phenotype in a salt marsh on Skidaway Island, Georgia, USA. FEMS Microbiology Ecology, 96:fiaa026.
Koop-Jakobsen K, Mueller P, Meier RJ, Liebsch G, Jensen K. Plant-sediment interactions in salt marshes–an optode imaging study of O2, pH, and CO2 gradients in the rhizosphere. Frontiers in plant science. 2018;9:541.
Koretsky CM, Moore CM, Lowe KL, Meile C, DiChristina TJ, Van Cappellen P. Seasonal oscillation of microbial iron and sulfate reduction in saltmarsh sediments (Sapelo Island, GA, USA). Biogeochemistry. 2003;64:179–203.
Kostka JE, Gribsholt B, Petrie E, Dalton D, Skelton H, Kristensen E. The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments. Limnology and Oceanography. 2002a;47:230–40.
Kostka JE, Roychoudhury A, Van Cappellen P. Rates and controls of anaerobic microbial respiration across spatial and temporal gradients in saltmarsh sediments. Biogeochemistry. 2002b;60:49–76.
Lee RW, Kraus DW, Doeller JE. Oxidation of sulfide by Spartina alterniflora roots. Limnology and Oceanography. 1999;44:1155–9.
Lever MA, Torti A, Eickenbusch P, Michaud AB, Šantl-Temkiv T, Jørgensen BB. A modular method for the extraction of DNA and RNA, and the separation of DNA pools from diverse environmental sample types. Frontiers in Microbiology. 2015;6:476.
Lim SJ, Davis BG, Gill DE, Walton J, Nachman E, Engel AS, et al. Taxonomic and functional heterogeneity of the gill microbiome in a symbiotic coastal mangrove lucinid species. The ISME journal. 2019a;13:902–20.
Lim SJ, Alexander L, Engel AS, Paterson AT, Anderson LC, Campbell BJ. Extensive thioautotrophic gill endosymbiont diversity within a single Ctena orbiculate (Bivalvia: Lucinidae) population and implications for defining host-symbiont specificity and species recognition. 2019b;mSystems, 4:e00280–19.
Lin L, Liu W, Zhang M, Lin X, Zhang Y, Tian Y. Different height forms of Spartina alterniflora might select their own rhizospheric bacterial communities in southern coast of China. Microbial Ecology. 2019;77:124–35.
Liu Y, Zhu A, Tan H, Cao L, Zhang R. Engineering banana endosphere microbiome to improve Fusarium wilt resistance in banana. Microbiome. 2019;7:74.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15:550.
Lovell CR, Piceno YM, Quattro JM, Bagwell CE. Molecular analysis of diazotroph diversity in the rhizosphere of the smooth cordgrass, Spartina alterniflora. Applied and Environmental Microbiology. 2000;66:3814–22.
Lundberg DS, Yourstone S, Mieczkowski P, Jones CD, Dangl JL. Practical innovations for high-throughput amplicon sequencing. Nature Methods. 2013;10:999–1002.
Maricle BR, Lee RW. Aerenchyma development and oxygen transport in the estuarine cordgrasses Spartina alterniflora and S. anglica. Aquatic Botany. 2002;74:109–20.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. Journal. 2011;17:10–2.
Martin BC, Alarcon MS, Gleeson D, Middleton JA, Fraser MW, Ryan MH, et al. Root microbiomes as indicators of seagrass health. FEMS Microbiology Ecology. 2020a;96(fiz201).
Martin BC, Middleton JA, Fraser MW, Marshall IP, Scholz VV, Schmidt H. Cutting out the middle clam: lucinid endosymbiotic bacteria are also associated with seagrass roots worldwide. The ISME journal. 2020b;14:2901–5.
McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one. 2013;8(4).
Mcowen CJ, Weatherdon LV, Van Bochove JW, Sullivan E, Blyth S, Zockler C, et al. A global map of saltmarshes. Biodiversity data journal. 2017;5:e11764.
Meier DV, Pjevac P, Bach W, Hourdez S, Girguis PR, Vidoudez C, et al. Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. The ISME Journal. 2017;11:1545–58.
Mendelssohn IA. The influence of nitrogen level, form, and application method on the growth response of Spartina alterniflora in North Carolina. Estuaries. 1979;2:106–12.
Mendelssohn IA, McKee KL. Indicators of environmental stress in wetland plants. In: Ecological indicators. Boston, MA: Springer; 1992. p. 603–24.
Mendelssohn IA, Morris JT. Eco-physiological controls on the productivity of Spartina alterniflora Loisel. In: Weinstein MP, Kreeger DA, editors. Concepts and controversies in tidal marsh ecology: Kluwer Academic Publishers; 2000. p. 59–80.
Mitsch WJ, Gosselink JG. Wetlands. New York, New York, USA: Van Nostrand Reinhold; 1993.
Morris JT, Dacey JWH. Effects of O2 on ammonium uptake and root respiration by Spartina alterniflora. American Journal of Botany. 1984;71:979–85.
Morris JT, Haley C, Krest R. Effects of sulfide concentrations on growth and dimethylsulphoniopropionate (DMSP) concentration in Spartina alterniflora. In: Kiene R, Visscher R, Keller M, Kirst G, editors. Biological and environmental chemistry of DMSP and related sulfonium compounds: Plenum; 1996. p. 87–95.
Moulana A, Anderson RE, Fortunato CS, Huber JA. Selection is a significant driver of gene gain and loss in the pangenome of the bacterial genus Sulfurovum in geographically distinct deep-sea hydrothermal vents. Msystems. 2020;5:e00673–19.
Murphy AE, Bulseco AN, Ackerman R, Vineis JH, Bowen JL. Sulphide addition favours respiratory ammonification (DNRA) over complete denitrification and alters the active microbial community in salt marsh sediments. Environmental Microbiology. 2020;22:2124–39.
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H., Wagner, H., Oksanen, M.J. Package ‘vegan’. Community ecology package, version. 2013;2(9):1–295.
Petersen JM, Kemper A, Gruber-Vodicka H, Cardini U, Van Der Geest M, Kleiner M, et al. Chemosynthetic symbionts of marine invertebrate animals are capable of nitrogen fixation. Nature microbiology. 2017;2:16195.
Peterson BJ. Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecologica. 1999;20:479–87.
Price MN, Dehal PS, Arkin AP. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucledic Acids Research. 2013;41:D590–6.
Ramírez DA, Yactayo W, Rens LR, Rolando JL, Palacios S, De Mendiburu F, et al. Defining biological thresholds associated to plant water status for monitoring water restriction effects: stomatal conductance and photosynthesis recovery as key indicators in potato. Agricultural Water Management. 2016;177:369–78.
Ramnarine R, Voroney RP, Wagner-Riddle C, Dunfield KE. Carbonate removal by acid fumigation for measuring the δ13C of soil organic carbon. Canadian Journal of Soil Science. 2011;91:247–50.
Simmons T, Caddell DF, Deng S, Coleman-Derr D. Exploring the root microbiome: extracting bacterial community data from the soil, rhizosphere, and root endosphere. Journal of Visualized Experiments. 2018;135:e57561.
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Applied and Environmental Microbiology. 2009;75:7537–41.
Shade A, Handelsman J. Beyond the Venn diagram: the hunt for a core microbiome. Environmental microbiology. 2012;14:4–12.
Sims GK, Ellsworth TR, Mulvaney RL. Microscale determination of inorganic nitrogen in water and soil extracts. Communications in Soil Science and Plant Analysis. 1995;26:303–16.
Spivak AC, Reeve J. Rapid cycling of recently fixed carbon in a Spartina alterniflora system: a stable isotope tracer experiment. Biogeochemistry. 2015;125:97–114.
Stegen JC, Lin X, Konopka AE, Fredrickson JK. Stochastic and deterministic assembly processes in subsurface microbial communities. The ISME journal. 2012;6:1653–64.
Stegen JC, Lin X, Fredrickson JK, Chen X, Kennedy DW, Murray CJ, et al. Quantifying community assembly processes and identifying features that impose them. The ISME journal. 2013;7:2069–79.
Stookey LL. Ferrozine - a new spectrophotometric reagent for iron. Anal. Chem. 1970;43:779–81.
Suzuki Y, Kojima S, Sasaki T, Suzuki M, Utsumi T, Watanabe H, et al. Host-symbiont relationships in hydrothermal vent gastropods of the genus Alviniconcha from the Southwest Pacific. Applied and Environmental Microbiology. 2006;72:1388–93.
Suzuki D, Li Z, Cui X, Zhang C, Katayama A. Reclassification of Desulfobacterium anilini as Desulfatiglans anilini comb. nov. within Desulfatiglans gen. nov., and description of a 4-chlorophenol-degrading sulfate-reducing bacterium, Desulfatiglans parachlorophenolica sp. nov. International journal of systematic and evolutionary microbiology. 2014;64:3081–6.
Thajudeen J, Yousuf J, Veetil VP, Varghese S, Singh A, Abdulla MH. Nitrogen fixing bacterial diversity in a tropical estuarine sediments. World Journal of Microbiology and Biotechnology. 2017;33:41.
Thomas F, Giblin AE, Cardon ZG, Sievert SM. Rhizosphere heterogeneity shapes abundance and activity of sulfur-oxidizing bacteria in vegetated salt marsh sediments. Frontiers in microbiology. 2014;5:309.
Tobias CR, Neubauer SC. Salt marsh biogeochemistry—an overview. In: Perillo GME, Wolanski E, Cahoon DR, Brinson MM, editors. Coastal wetlands: an integrated ecological approach: Elsevier; 2019. p. 445–92.
Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, et al. Core microbiomes for sustainable agroecosystems. Nature Plants. 2018;4:247–57.
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. Plant–microbiome interactions: from community assembly to plant health. Nature Reviews Microbiology. 2020;18:607–21.
Valiela I, Teal JM, Deuser WG. The nature of growth forms in the salt marsh grass Spartina alterniflora. The American Naturalist. 1978;112:461–70.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology. 2007;73:5261–7.
Watanabe FS, Olsen SR. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Science Society Proceedings. 1965;29:677–8.
White JF, Kingsley KL, Zhang Q, Verma R, Obi N, Dvinskikh S, et al. Endophytic microbes and their potential applications in crop management. Pest management science. 2019;75:2558–65.
Whiting GJ, Gandy EL, Yoch DC. Tight coupling of root-associated nitrogen fixation and plant photosynthesis in the salt marsh grass Spartina alterniflora and carbon dioxide enhancement of nitrogenase activity. Applied and Environmental Microbiology. 1986;52:108–13.
Wieski K, Pennings S. Climate drivers of Spartina alterniflora saltmarsh production in Georgia, USA. Ecosystems. 2014;17:473–84.
Zhang L, Zhang W, Li Q, Cui R, Wang Z, Wang Y, et al. Deciphering the root endosphere microbiome of the desert plant Alhagi sparsifolia for drought resistance-promoting bacteria. Applied and Environmental Microbiology. 2020;86:e02863–19.
Zheng Y, Hou L, Liu M, Yin G, Gao J, Jiang X, et al. Community composition and activity of anaerobic ammonium oxidation bacteria in the rhizosphere of salt-marsh grass Spartina alterniflora. Applied Microbiology and Biotechnology. 2016;100:8203–12.
Zogg GP, Travis SE, Brazeau DA. Strong associations between plant genotypes and bacterial communities in a natural salt marsh. Ecology and Evolution. 2018;8:4721–30.
Acknowledgements
The authors would like to acknowledge Christina Stoner and Jack Cenatempo, as well as teachers from the GCE Schoolyard Program, for their fieldwork assistance. This is contribution 1087 of the University of Georgia Marine Institute.
Funding
This work was supported in part by an institutional grant (NA18OAR4170084) to the Georgia Sea Grant College Program from the National Sea Grant Office, National Oceanic and Atmospheric Administration, US Department of Commerce, and by a grant from the National Science Foundation (DEB 1754756). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Contributions
J.L.R., M.K., and J.E.K conceived of the study; J.L.R., M.K., T.S., and J.E.K collected samples from the field; J.L.R. and M.K. performed the experiment and the data analyses. J.L.R., M.K., and J.E.K. wrote the manuscript; J.L.R., M.K. T.S., and J.E.K. provided valuable insight and ideas during numerous sessions of discussion. All authors provided critical comments on the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1: List of genera containing species with known sulfate reducing, sulfur oxidizing, iron oxidizing, and nitrifying activity.
Additional file 2.
Tables S2–S4 and Figs. S1–S8
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rolando, J.L., Kolton, M., Song, T. et al. The core root microbiome of Spartina alterniflora is predominated by sulfur-oxidizing and sulfate-reducing bacteria in Georgia salt marshes, USA. Microbiome 10, 37 (2022). https://doi.org/10.1186/s40168-021-01187-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40168-021-01187-7